Additions and corrections
Engineering of spectator glycocalyx structures to evaluate molecular interactions at crowded cellular boundaries
Daniel J. Honigfort, Michelle H. Zhang, Stephen Verespy, III, and Kamil Godula*
Faraday Discuss., 2019, 219, 138-153 (DOI: 10.1039/C9FD00024K). Amendment published 13 March 2020.
The authors would like to thank a reader for bringing to the attention an error in one of the figures in the above manuscript. In Fig. 2A of the manuscript, the drawing of the alkynyl cholestanone molecule 2 contained an extra ring that is not present in the molecule used as the hydrophobic anchor based on ref. 20.
A corrected figure drawing is provided below as Fig. 1, and the proton and carbon spectra for compound 2 used in the study as Fig 2 and Fig. 3 respectively. The NMR signals for compound 2 synthesized by the authors are a match to the published data for this compound from ref. 20. The data and conclusions in the paper are not affected by this error.

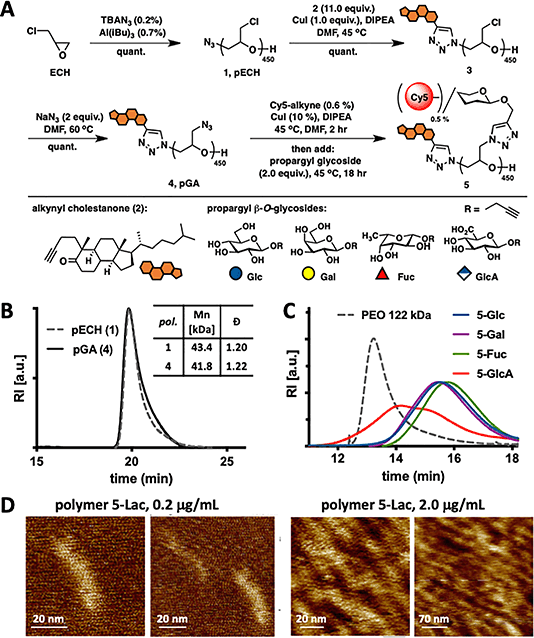
Fig. 1: Synthesis and characterization of mucin mimetics 5. (A) Glycopolymers 5 were generated from poly(epichlorohydrin) 1 via chloride-to-azide sidechain substitution followed by the CuAAC conjugation of propargyl β-O-glycosides (Glc, Gal, Fuc, and GlcA). The polymers were furnished with a hydrophobic cholestanone moiety for anchoring into cell membranes and a Cy5 fluorescent tag for imaging and quantification. (B) SEC analysis indicated narrow molecular weight and chain-length distributions before and after the chloride-to-azide exchange. (C) Glycopolymers 5 exhibited increased retention in aqueous SEC compared to a PEO standard of similar molecular weight (122 kDa), a characteristic behavior of glycopolymers with extended molecular conformations. (D) AFM imaging of lactose-modified glycopolymers confirmed elongated, mucin-like morphology of the PEG-based glycopolymers.
Fig. 2: 1H NMR (300 MHz, CDCl3) of alkynyl-cholestanone 2.
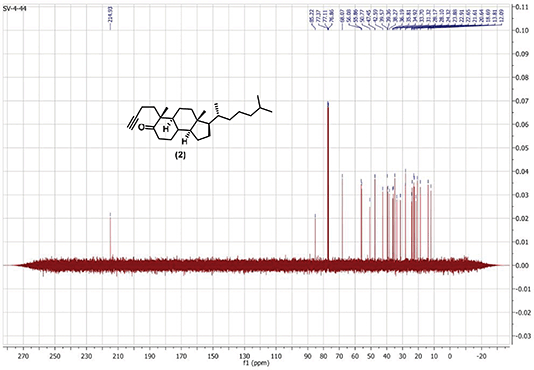
Fig. 3: 13C NMR (500 MHz, CDCl3) of alkynyl-cholestanone 2.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article