Additions and corrections
Oxidative steam reforming of methanol over CuZnAl(Zr)-oxide catalysts; a new and efficient method for the production of CO-free hydrogen for fuel cells
S. Velu, K. Suzuki and T. Osaki
Chem. Commun., 1999, 2341. Amendment published 17th January 2000
Kindly note that Table 2 and Fig. 1(B) (H2 production rate) in the original paper should read as given below:
Table 2 Performance of various CuZnAl(Zr)-oxide catalysts in the oxidative steam reforming of methanol after 25 h of on-stream operation at 230 °C; MeOH injection rate = 83 mmol kg(catalyst)-1 s-1
| Catalyst | MeOH conversion | H2 production rate/mmol kg-1 s-1 | H2 production rate/MeOH conversion rate | Carbon selectivity (mol%) | ||
| mol% | Rate/mmol kg-1 s-1 | CO | CO2 | |||
| CZAZ-A | 37.6 | 31 | 65 | 2.1 | 0.0 | 100 |
| CZAZ-B | 65.4 | 54 | 128 | 2.4 | 0.0 | 100 |
| CZAZ-C | 68.1 | 57 | 156 | 2.7 | 0.0 | 100 |
| CZAZ-Ca | 100.0 | 53 | 126 | 2.4 | 0.0 | 100 |
| CZAZ-D | 79.6 | 66 | 171 | 2.6 | 1.2 | 98.8 |
| CZAZ-E | 85.4 | 71 | 185 | 2.6 | 1.1 | 98.9 |
| CZAZ-F | 90.0 | 75 | 194 | 2.6 | 0.8 | 99.2 |
a MeOH injection rate = 53 mmol kg(catalyst)-1 s-1.

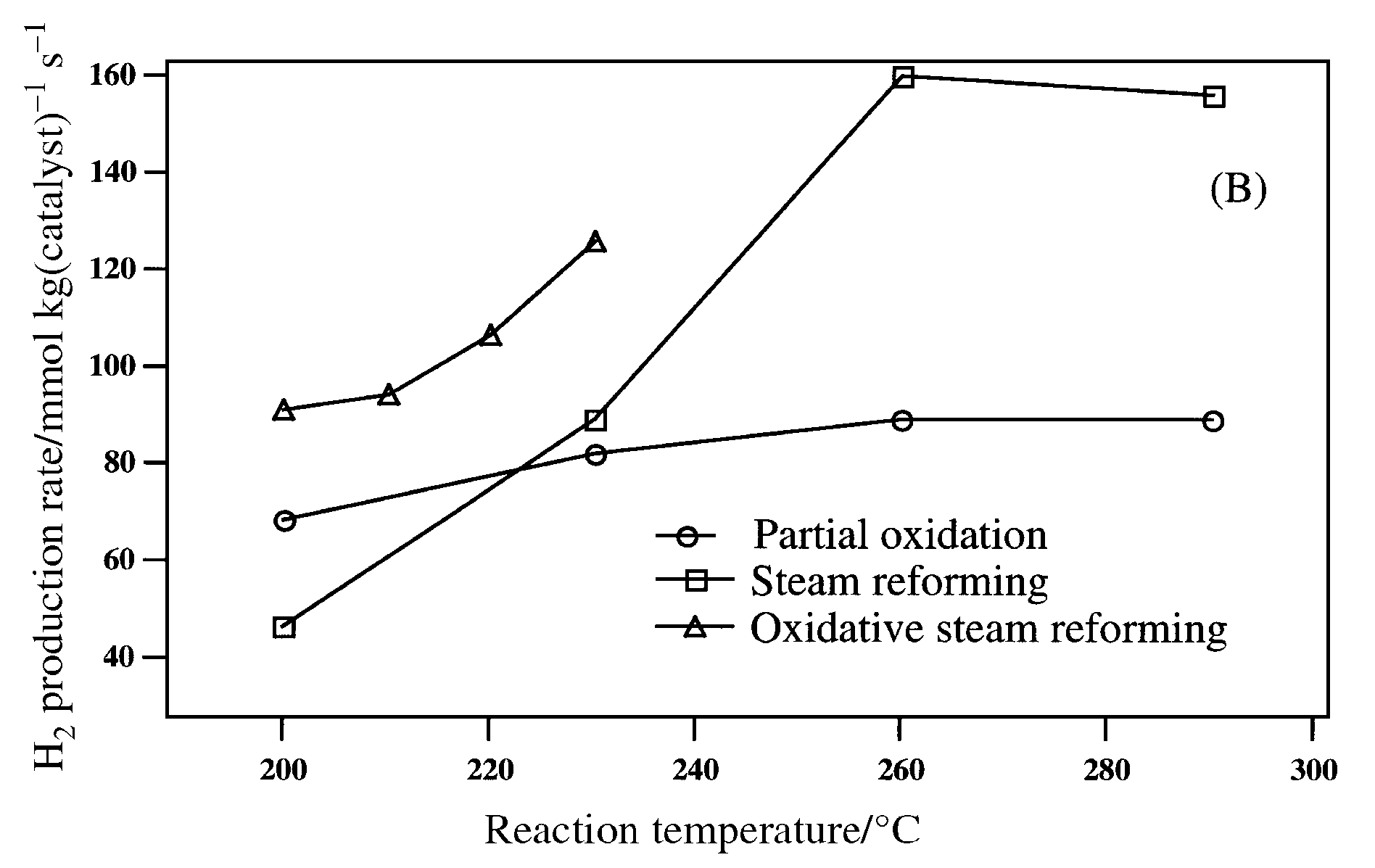
Fig. 1 (B) Effect of temperature in catalytic performance of CZAZ-C catalyst in the partial oxidation, steam reforming, and oxidative steam reforming of methanol; H2 production rate.
Thanks are due to Dr. Hung-Wen Jen, Chemical Engineering Department, Ford Research Laboratory, Ford Motor Company, P.O. Box 2503, Dearborn, MI 48121, USA, for pointing out the errors in the rate data calculations in the original paper.