Additions and corrections
Synthesis of 1,3-dioxo-hexahydropyrido[1,2-c][1,3]diazepine carboxylates, a new bicyclic skeleton formed by ring expansion-RCM methodology
Nicolai Dieltiens, Diederica D. Claeys, Bart Allaert, Francis Verpoort and Christian V. Stevens
Chem. Commun., 2005, 4477-4478 (DOI: 10.1039/b508663a) Amendment published 15th December 2005
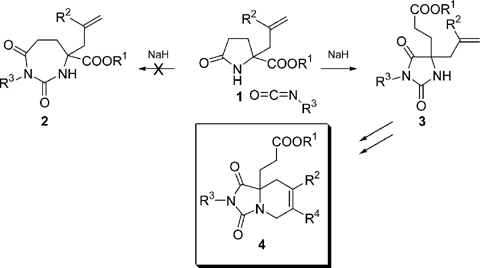
An incorrect product was reported in this article. After publication, X-ray analysis showed that the products formed by reaction of pyroglutamates 1 with isocyanates are not perhydro-1,3-diazepine-2,4-diones 2, but are hydantoins 3 which are formed by a ring closing-ring opening sequence and which have almost identical NMR spectra. As a consequence, the RCM products synthesised (Scheme 3) are in fact bicyclic hydantoin derivatives 4. A full paper is in preparation stating this misinterpretation and proving the structure of the hydantoins by X-ray analysis.

The Royal Society of Chemistry apologises for this error and any consequent inconvenience to authors and readers.
Back to article