Additions and corrections
Synthesis of cytotoxic ferrocenyl flavones via a ferricenium-mediated 1,6-oxidative cyclization
Jean-Philippe Monserrat, Guy G. Chabot, Louis Hamon, Lionel Quentin, Daniel Scherman, Gérard Jaouen and Elizabeth A. Hillard
Chem. Commun., 2010, 5145-5147 (DOI: 10.1039/c0cc01290d) Amendment published 12th August 2010
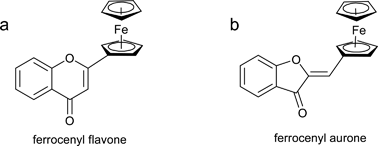
In the article cited above, we reported the first synthesis and antiproliferative effects of what we characterized as ferrocenyl flavones of the type 2-ferrocenyl-chromen-4-one (Chart 1). However, a recent X-ray crystal structure of one of the reported compounds (2c, crystals obtained from dichloromethane/petroleum ether) revealed it to be a structural isomer, the aurone (Z)-5-bromo-2-(ferrocenylidene)benzofuran-3-one (Figure 1). Only one isomer was observed in NMR, and X-ray crystallographic analysis shows it to be the Z-isomer. Thin layer chromotography of the crystalline sample used in X-ray analysis indicated its purity. Based on the uniformity of the synthesis, the color and the 1H and 13C NMR spectra of the compounds, all compounds reported as flavones are now believed to be aurones of the type 2-(ferrocenylidene)benzofuran-3-one (Chart 1).

Chart 1. a) Previously proposed general ferrocenyl flavone structure; b) Corrected general ferrocenyl aurone structure.
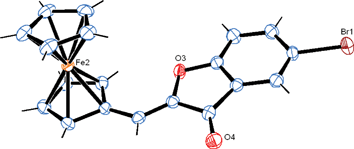
Figure 1. Ortep diagram of the ferrocenyl aurone, (Z)-5-bromo-2-(ferrocenylidene)benzofuran-3-one. Crystallographic data (excluding structure factors) for the structure has been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 787468. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44 (0)1223 336033 or e-mail: deposit@ccdc.cam.ac.uk).
Therefore, the corrected Scheme 1 is as follows.
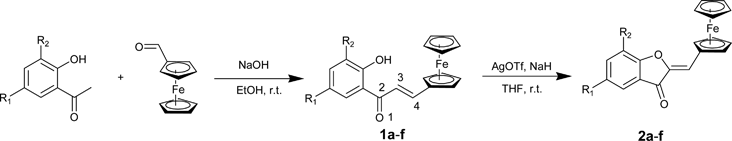
Scheme 1. a: R1=R2=H; b: R1=Cl, R2=H; c: R1=Br, R2=H; d: R1=R2=Cl; e: R1=R2=Br; f: R1=R2=F
Taking into account the corrected structure, the proposed mechanism is proven incorrect. Instead, there is clearly an attack on the 3 position of the ketone to form a five-membered ring. Although AgOTf oxidizes the ferrocenyl moiety when added before the base (NaH), we have no evidence that oxidation of ferrocene occurs in basic media. Instead of the 1,6 oxidative cyclization referred to in the title, we observe a 1,5 oxidative cyclization.
This structural reassignment incurs a slight change in the assignment of the 13C NMR signals (as given in the supporting information); specifically the chemical shift of one aromatic carbon and the tertiary vinylic carbon are switched:
2a. δC(75 MHz; CDCl3; Me4Si) 182.9 (Cketone), 165.4 (Car), 146.0 (Cvinyl), 136.1 (Car), 124.5 (Car), 123.1 (Car), 122.6 (Car), 116.4 (Cvinyl), 112.9 (Car), 75.1 (C5H4, Cq), 71.8 (C5H4), 71.5 (C5H4), 70.0 (C5H5).
2b. δC(75 MHz; CDCl3; Me4Si) 181.4 (Cketone), 163.5 (Car), 146.1 (Cvinyl), 135.8 (Car), 128.8 (Car), 124.0 (Car), 123.8 (Car), 117.9 (Cvinyl), 114.2 (Car), 74.7 (C5H4, Cq), 72.2 (C5H4), 71.6 (C5H4), 70.0 (C5H5).
2c. δC(75 MHz; CDCl3; Me4Si) 181.2 (Cketone),163.9 (Car), 145.9 (Cvinyl), 138.5 (Car), 127.1 (Car), 124.4 (Car), 118.0 (Cvinyl), 115.9 (Car), 114.7 (Car),74.7 (C5H4, Cq), 72.2 (C5H4), 71.7 (C5H4), 70.1 (C5H5).
2d. δC(75 MHz; CDCl3; Me4Si) 179.3 (Cketone), 158.2 (Car), 144.8 (Cvinyl), 134.0 (Car), 127.9 (Car), 124.1 (Car), 121.4 (Car), 118.9 (Cvinyl), 118.4 (Car), 73.3 (C5H4, Cq), 71.7 (C5H4), 71.0 (C5H4), 69.2 (C5H5).
2e. δC(75 MHz; CDCl3; Me4Si) 179.3 (Cketone), 159.9 (Car), 144.4 (Cvinyl), 139.2 (Car), 129.8 (Car), 125.0 (Car), 124.5 (Car), 119.0 (Cvinyl), 115.0 (Car), 73.3 (C5H4, Cq), 71.7 (C5H4), 71.0 (C5H4), 69.2 (C5H5).
2f. δC(100 MHz; CDCl3; Me4Si) 180.7 (Cketone), 157.9 (dd, 1J=246.5 Hz, 3J=7.3 Hz, Car), 148.8 (d, 2J=11.3 Hz, Car), 148.1 (dd, 1J=254.7 Hz, 3J=11.2 Hz, Car), 145.9 (Cvinyl), 125.6 (d, 3J=7.7 Hz, Car), 119.8 (Cvinyl), 110.9 (dd, 2J=19.7 Hz, 2J=24.3 Hz, Car), 105.5 (dd, 2J=23.8 Hz, 4J=4.1 Hz, Car), 74.3 (C5H4, Cq), 72.6 (C5H4), 71.9 (C5H4), 70.2 (C5H5).
The authors acknowledge P. Herson for the crystal structure.
The Royal Society of Chemistry apologises for this error and any consequent inconvenience to authors and readers.
Back to article