Additions and corrections
Cation distribution in cubic NaM(PO3)3 (M = Mg or Zn) using X-ray powder diffraction and solid state NMR
Isaac Abrahams, Aliya Ahmed, Christopher J. Groombridge, Geoffrey E. Hawkes and Teresa G. Nunes
J. Chem. Soc., Dalton Trans., 2000, 155. Amendment published 3rd February 2000
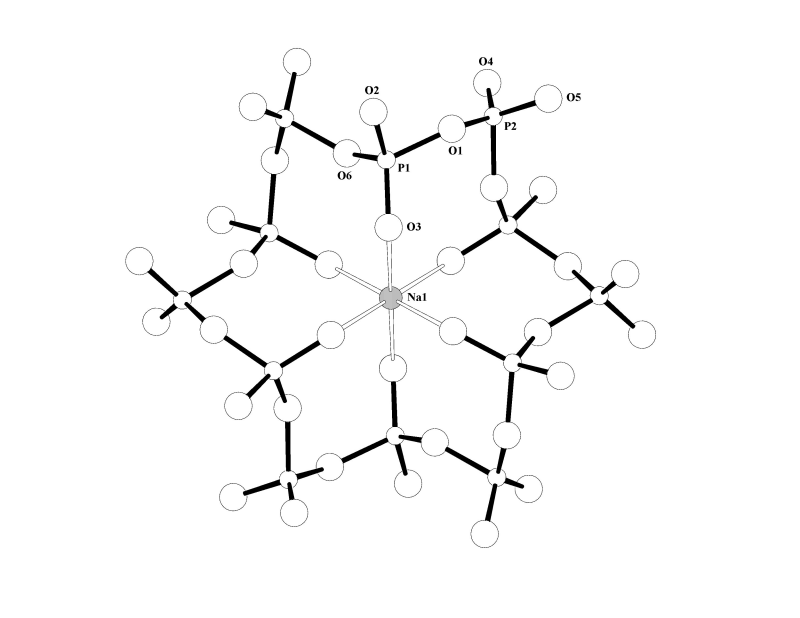
The structures of NaMg(PO3)3 and NaZn(PO3)3 were incorrectly described as containing polyphosphate chains, (PO3)nn-. In fact their structures are built from polyphosphate rings which constitute the cyclic anion (P12O36)12-. The structural formulae are therefore best described as Na4Mg4(P12O36) and Na4Zn4(P12O36). The anion rings are convoluted with the non-bridging O(3) atoms pointing to the centre of the ring creating a distorted octahedral coordination environment for the central Na(1) ion (Fig. 1). The other cations are accommodated in distorted octahedral cavities between the rings.

Fig. 1 Coordination of Na(1) at the centre of the cyclic (P12O36)12- anion in NaM(PO3)3 (M = Mg or Zn).