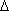 G°rxn should read +1.37 kcal mol-1 and not -1.42 kcal mol-1. As a result
G°rxn should read +1.37 kcal mol-1 and not -1.42 kcal mol-1. As a result 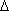 H°acid for benzocyclobutanone is 359.8 kcal mol-1 and not 360.3 kcal mol-1.
H°acid for benzocyclobutanone is 359.8 kcal mol-1 and not 360.3 kcal mol-1.Additions and corrections
Benzocyclobutenone enolate: an anion with an antiaromatic resonance structure
Katherine M. Broadus and Steven R. Kass
J. Chem. Soc., Perkin Trans. 2, 1999, 2389
On page 2391, line 12, 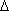 G°rxn should read +1.37 kcal mol-1 and not -1.42 kcal mol-1. As a result
G°rxn should read +1.37 kcal mol-1 and not -1.42 kcal mol-1. As a result 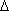 H°acid for benzocyclobutanone is 359.8 kcal mol-1 and not 360.3 kcal mol-1.
H°acid for benzocyclobutanone is 359.8 kcal mol-1 and not 360.3 kcal mol-1.
In eqn. (16), 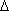 H°rxn = -7.4 ± 5 kcal mol-1 not -6.9 ± 5 kcal mol-1. Benzocyclobutenone is a stronger acid than cyclobutanone by 7.4 kcal mol-1 rather than 6.9 kcal mol-1 as originally reported.
H°rxn = -7.4 ± 5 kcal mol-1 not -6.9 ± 5 kcal mol-1. Benzocyclobutenone is a stronger acid than cyclobutanone by 7.4 kcal mol-1 rather than 6.9 kcal mol-1 as originally reported.
In eqn. (17), 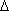 H°rxn = -9.6 ± 3 kcal mol-1 not -10.1 ± 3 kcal mol-1. 1-Phenylpropan-2-one is more acidic than benzocyclobutenone by 9.6 ± 3 kcal mol-1 rather than 10.1 ± 3 kcal mol-1 as originally reported.
H°rxn = -9.6 ± 3 kcal mol-1 not -10.1 ± 3 kcal mol-1. 1-Phenylpropan-2-one is more acidic than benzocyclobutenone by 9.6 ± 3 kcal mol-1 rather than 10.1 ± 3 kcal mol-1 as originally reported.