Shapeshifting molecules
Picture: © Shutterstock
ChemSci Pick of the Week
Scientists in the UK have investigated the phenomenon of shape-selectivity in shape-shifting molecules – molecules that adopt different configurations depending on their environment.
Most synthetic materials we make and use – such as drugs, plastics or dyes – are based on molecules with fixed structures. Molecules are made up of atoms joined to one another by a series of chemical bonds, and although many molecules can bend or twist to some extent, the atoms are held together in essentially fixed arrangements.
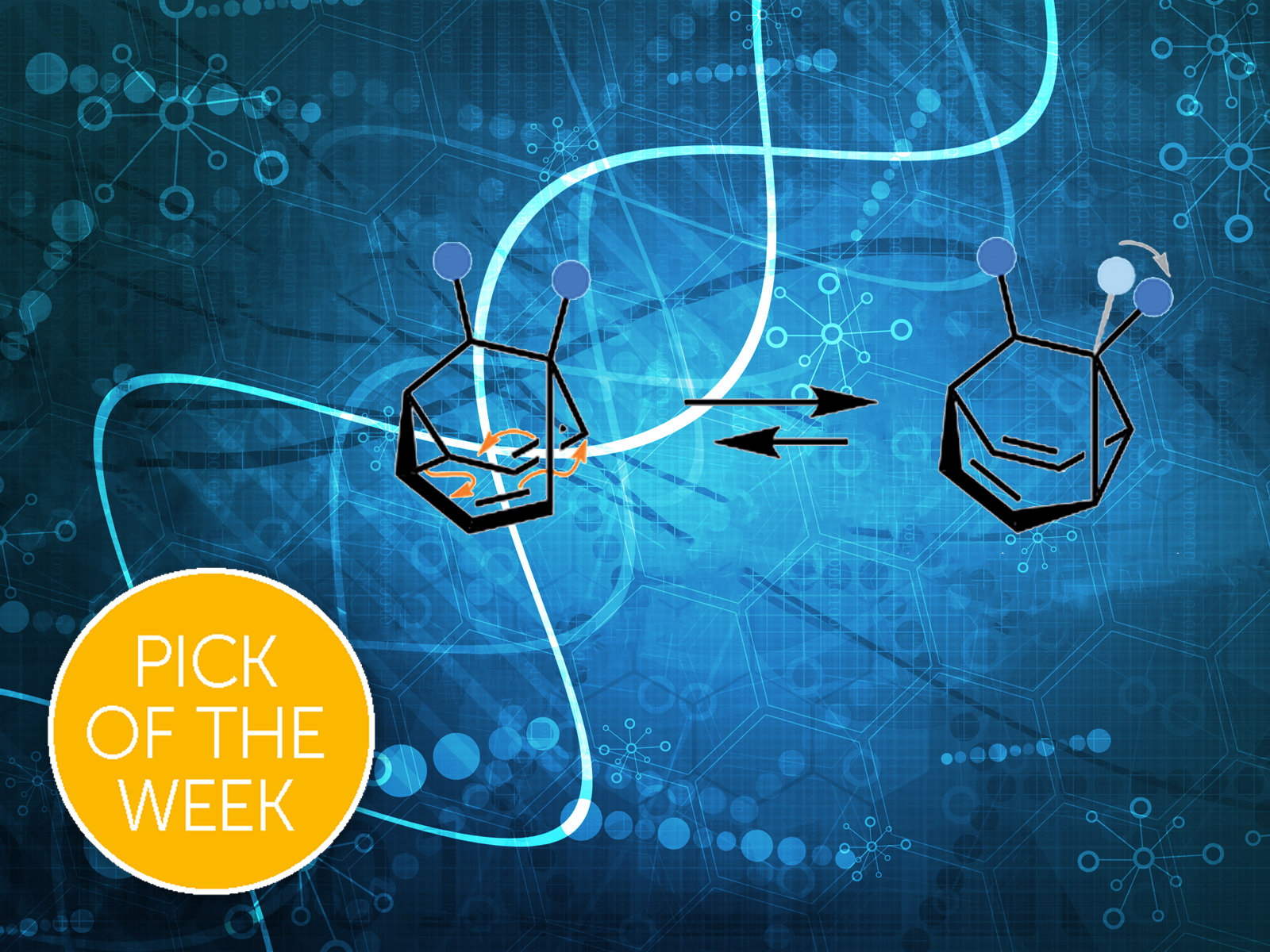
Dr Paul McGonigal from Durham University investigates a class of molecules that have ‘shapeshifting’ properties. In other words they can totally change their shape in response to their environment. Dr McGonigal’s group have looked at the specific scenario where the molecules are dissolved a solution, and then crystallise out into different formations. They have discovered a shape-shifting molecule that can crystallise into an unexpected conformation.
He explains: "The shapeshifting molecules described in this paper are like an unruly game of musical chairs. When the molecules are dissolved, it’s like the music is playing – the atoms in the structure switch places with one another and the overall shape of the molecule changes back and forth."
"For now, we have investigated what happens when the molecules form crystals, which is like the music stopping. Just like the players taking their seats, the atoms are no longer mobile and the overall structure becomes fixed. And just as it is difficult to predict where players will end up in a game of musical chairs, our experiments gave unexpected results. By studying these outcomes, we have started to learn about the factors that control this game of molecular musical chairs, i.e., what controls the preference for one of the rearranged molecules over another."
What makes this more interesting is that in the case of these special molecules, the environment can cause the less-favoured configuration of the molecule to form. Normally one configuration is more stable than another, but in this instance the less stable configuration can form, given the right conditions.
Crystallisation is one obvious way in which the shape of a molecule can become fixed. However there are other possibilities. For example a drug molecule may adapt its structure to match the active site on an enzyme. Dr McGonigal’s work relates to the rather simple case of crystals, but he believes that this could lead to greater understanding of more complex environments.
"With more time, we will be able to apply this knowledge to understand how the molecules respond in more complex, messy environments (e.g. inside a biological cell), that are more likely to encountered in real life."
This article is free to read in our open access, flagship journal Chemical Science: Aisha Bismillah et al., Chem. Sci., 2018, Advance Article. DOI: 10.1039/C8SC04303E. You can access all of our ChemSci Picks in this article collection.
Read more like this