Polymers for life

Picture: © Shutterstock
ChemSci Pick of the Week
Scientists have used a technique called living polymerisation to create high-quality polymers that could be used in solar cells transistors, and other electronic devices.
Polymers – long-chain molecules – are synthesised by stringing together olefins – smaller molecules containing double or triple bonds.
It sounds simple, but in practice there are a lot of ways it can go wrong. One particular challenge is that of selectivity. Imagine threading beads onto a string. The beads can go on in different orientations – how do we control the reaction so that all the beads string together in the same orientation?
Another challenge is how to avoid premature termination of the chains. The goal of polymerisation is normally to make very long chains, all of a very similar length – otherwise described as high molecular weight and low polydispersity. But if the forming chains terminate too early we end up with a collection of different length chains, including some very short chains. This type of product is much less useful for creating new materials. One way that termination occurs is by the ends of two growing polymer chains meeting each other by accident, leaving us with a short chain that can no longer grow.
In today’s pick of the week, we look at some ruthenium-based catalysts developed by a team of scientists at Seoul National University and California Institute of Technology. These catalysts build on third-generation Grubbs catalysts – named after Robert H. Grubbs, the chemist who supervised their synthesis, and Professor Grubbs himself is a contributor to the paper.
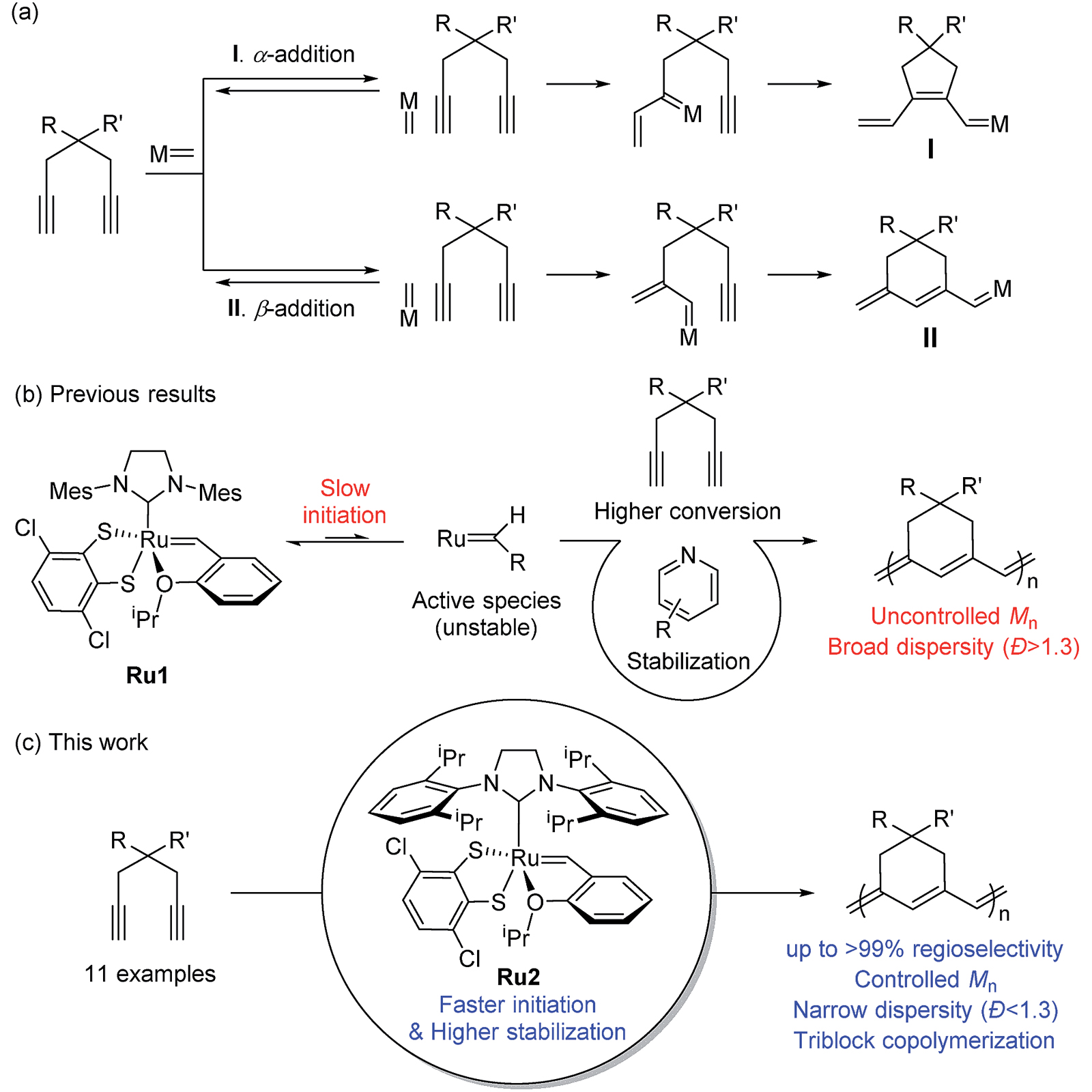
A scheme from the paper, showing how polymer chains of six-membered rings can be synthesised from 1,6-diynes, using a ruthenium-based catalyst Picture: © Royal Society of Chemistry
The new catalysts are able to achieve polymerisation with high selectivity, high molecular weight and low polydispersity. The starting monomers are 1,6-diynes – chains of seven carbon atoms including a triple bond at each end. These can form chains of either five- or six-membered rings, but the new catalyst is designed in such a way as to ensure six-membered rings are formed exclusively. This is one of very few instances in which this has been achieved.
By using a catalyst with very bulky ligands, the researchers have prevented chains from meeting each other and terminating prematurely, meaning polymerisation keeps going until a terminating molecule is deliberately added. This is known as 'living polymerisation'.
Finally, the researchers have used this method to create diblock copolymers – chains consisting of one type of monomer followed by a different type. Returning to the bead analogy, imagine a string where the first half is threaded exclusively with one shape of bead, and the second half is threaded with a different shape of bead.
Corresponding author Dr Tae-Lim Choi says: "This method lets us obtain semi-conducting materials with well-defined structures and fewer defects. This would hopefully lead to better electronic materials with higher performance in organic-based devices such as solar cells and transistors."
This work is expected to contribute to the development of more and better semi-conducting polymers, which would ultimately lead to making electronics lighter, more flexible and easier to carry.
This article is free to read in our open access, flagship journal Chemical Science: Tae-Lim Choi et al., Chem. Sci., 2019, Advance Article. DOI: 10.1039/C9SC01326A. You can access our 2019 ChemSci Picks in this article collection.
Read more like this